Human Specimens and/or Data
The latest formset applications involving the use of human specimens or data may not be considered to be human subjects research, depending on the details of the materials to be used. To help determine whether your research is classified as human subjects research, refer to the NIH Research Involving Private Information or Biological Specimens flowchart.
Forms-E proposals that don’t fit the definition of human subjects research, but use unidentifiable specimens or data, must include a narrative justification for the claim that no human subjects are involved. This narrative should ONLY be used if the project does NOT involve human subjects per the above guidance.
For proposals using data and/or specimens (not human subjects), the Coeus questionnaire should be answered “NO” to human subjects, and “YES” to the follow up question about specimens and/or data:
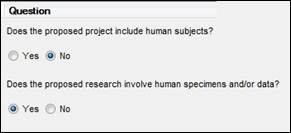
Per the NIH guidance, “If you answer ‘Yes’ to the ‘Does the proposed research involve human specimens and/or data?’ question, you must provide a justification for your claim that no human subjects are involved. This justification should include:
- information on who is providing the data/biological specimens and their role in the proposed research;
- a description of the identifiers that will be associated with the human specimens and data;
- a list of who has access to subjects’ identities; and
- information about the manner in which the privacy of research participants and confidentiality of data will be protected.”
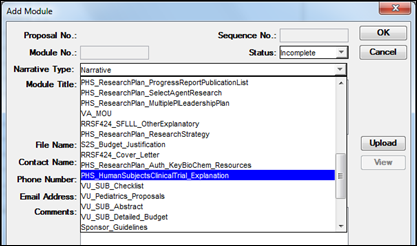
The justification is uploaded into Coeus under the Narratives section using Narrative Type PHS_HumanSubjectsClinicalTrial_Explanation.
Proposals involving human specimens and/or data that are not considered human subjects research do not need to use the eCat tool or include a human subjects special review in Coeus.
As always, the Funding Opportunity Announcement (FOA) may contain instructions that supersede the general guidance described above. Carefully review your FOA and consult your OSP specialist regarding any questions you may have.
Additional Resources:
- If the investigator decides to answer “Yes” to human subjects, the eCat tool for human subjects data includes detailed guidance concerning each section and question.
- For instructions on human subjects proposals utilizing Exemption 4 with the latest formsets, see our article on the topic.
- For more detailed guidance concerning human subjects definitions, see the NIH Human Subjects FAQ.
